
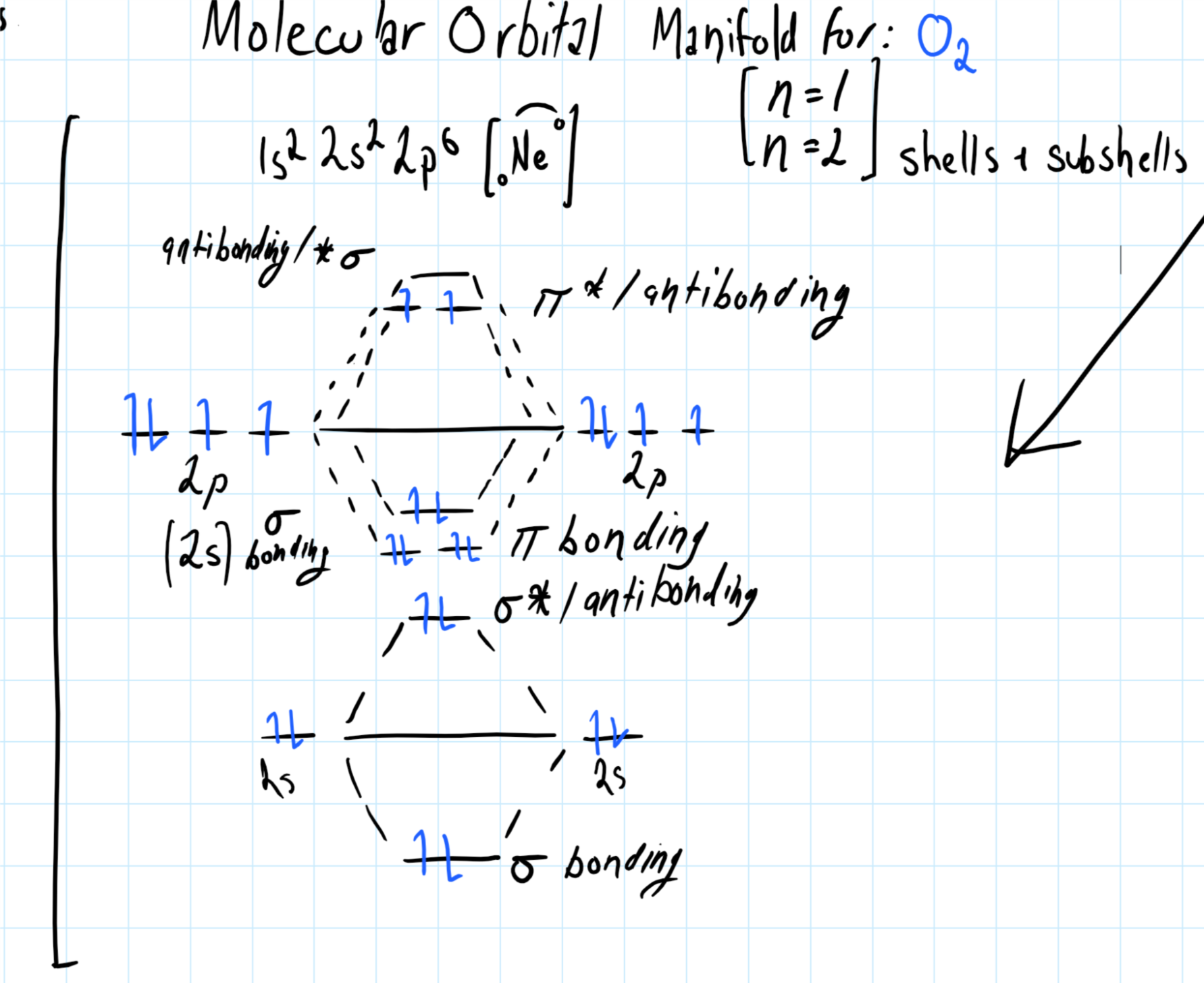
In SO42-, if you compare the electronegativities of Sulphur and Oxygen atoms, Oxygen is more electronegative than Sulphur atoms. This doesn’t apply to Hydrogen atoms, as these atoms never take the central position. To determine which atom takes the central atom, always remember that the less electronegative atom takes the central position, and more electronegative atoms are arranged around it. Here as we already have the total number of valence electrons, we can start by determining the central atom. Arrangement of the atoms in the molecule.There are some exceptions to this rule certainly, but the majority of the atoms follow this rule.įor determining the Lewis Structure for SO42- molecule, we need to know the feeling: Generally, while forming bonds with other atoms, every atom tries to follow an octet rule that states that an atom must have eight valence electrons to attain a stable structure. Apart from that, it also helps to know the molecular shape, polarity, and other properties of the molecule. The Lewis Structure of any molecule helps to understand the bonding of atoms in the structure.
Hence there are a total of 32 valence electrons for the Sulfate ion. Total valence electrons for SO42- = 6 + 6(4)+2 = 32 Here we have one Sulphur atom and 4 Oxygen atoms, and both these elements belong to the same group and have six valence electrons in their outermost shell.īut as there is a -2 charge on this molecule, it means that it accepts two additional electrons to attain a stable structure, and hence we have to consider these electrons as well. These electrons are the ones that are present in the outermost shell of the atom and participate in forming bonds. Concluding Remarks SO42- Valence electronsįor determining the Lewis Structure for any molecule, we first need to know the total number of valence electrons.


 0 kommentar(er)
0 kommentar(er)
